ABSTRACT
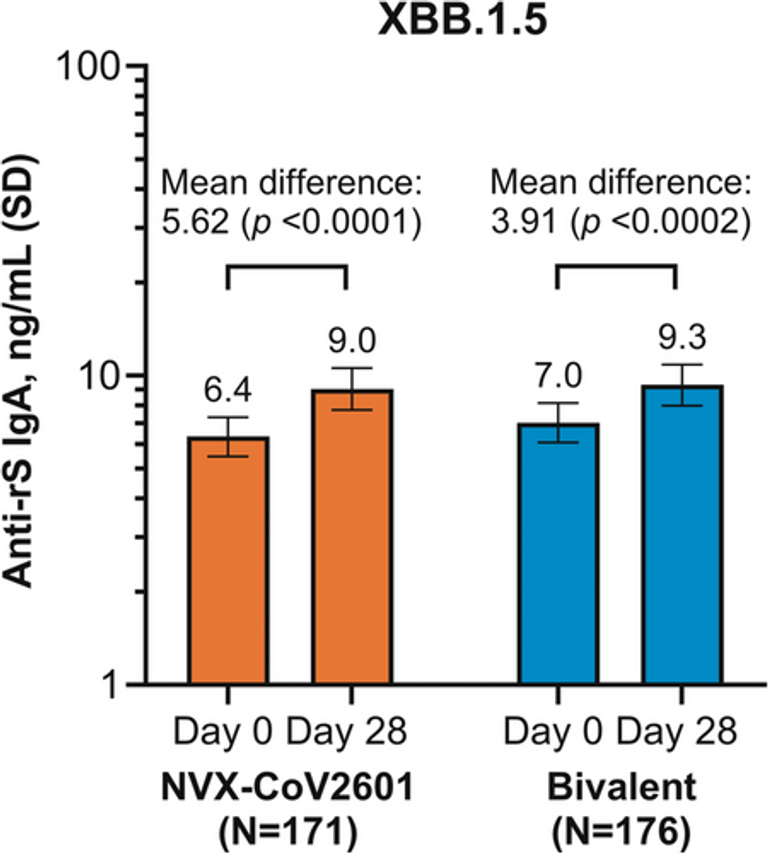
Immunoglobulin (Ig) A acts as a first line of defense against respiratory pathogens. Mucosal IgA in salivary and nasal passages has a rapid response to antigens and can play a protective role against reinfection. The mainstay for analyzing SARS-CoV-2 infection and vaccine efficacy has been assessment of serum IgG levels; however, validated assays for assessment of mucosal IgA in clinical samples are necessary as new and adapted measures are generated to combat immune-evasive viral variants. A mucosal IgA assay was developed and tested through assessment of IgA levels in salivary samples from participants of the 2019nCoV-314/NCT05973006 study. These participants had previously received ≥ 2 mRNA-based COVID-19 vaccinations prior to enrollment and received a single intramuscular study dose of NVX-CoV2601 (XBB.1.5) or bivalent vaccine (NVX-CoV2601 + NVX-CoV2373 [Wuhan]). Salivary samples were collected prior to vaccination on Day 0 and on Day 28 to assess response postvaccination. Both vaccine groups elicited a significant increase in anti-SARS-CoV-2 spike IgA against XBB.1.5. Furthermore, cross-reactivity by identification of anti-JN.1 and anti-Wuhan IgA was also observed. The detection of IgA in clinical mucosal samples using this assay will be a valuable tool in supporting vaccine development.