ABSTRACT
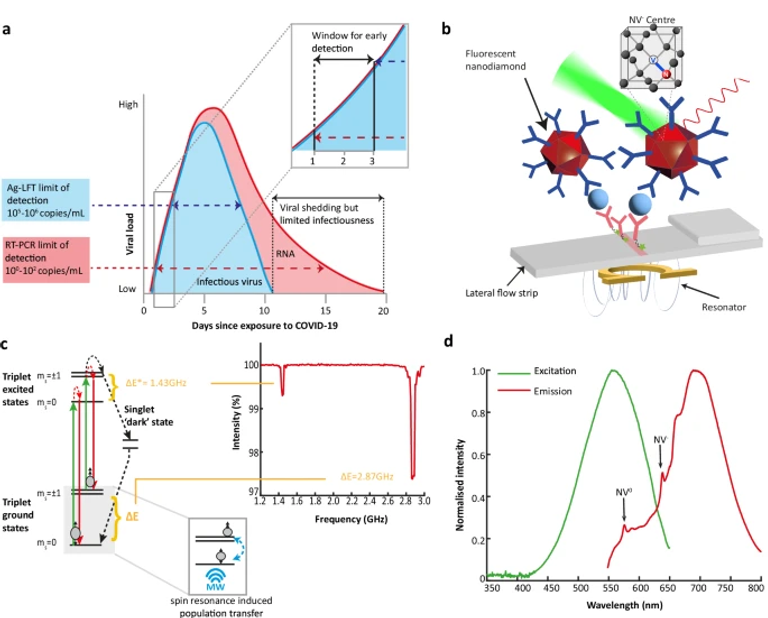
Quantum biosensors, which harness quantum effects to detect biomarkers, could address the urgent need for more sensitive rapid diagnostics. Lateral flow tests using nitrogen-vacancy centres in nanodiamond labels offer high sensitivity and robustness by controlling the spin-dependent fluorescence to remove background. This is particularly important in complex and variable clinical samples. However, to date only model systems have been studied with few clinical samples. Here we show results of a clinical evaluation of a spin-enhanced nanodiamond test for SARS-CoV-2 antigen with 103 upper respiratory tract swab samples. We find 95.1% sensitivity (Ct ≤ 30) and 100% specificity benchmarked against RT-qPCR, with no cross-reactivity to influenza A, RSV, and Rhinovirus. Modelling with patient data yields a mean of 2.0-days earlier detection compared to conventional gold-nanoparticle tests (just 0.6 days after RT-qPCR) with 2.2-fold more patients detected on the first day of symptom onset, potentially reducing the transmission risk and protecting populations.